
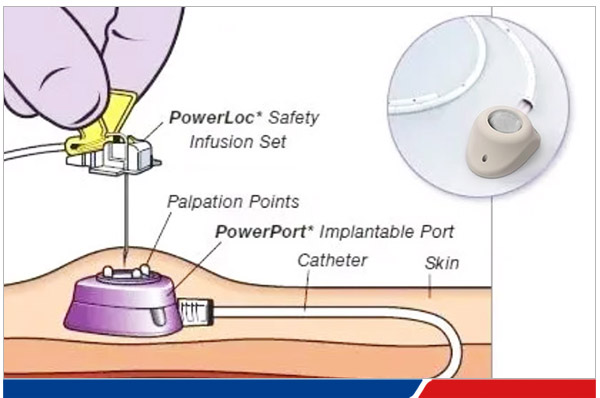
What is a PORT
The infusion port (PORT), also known as the implantable venous catheter system, is a closed venous infusion system that can be completely implanted in the body. 80% of them are used for cancer treatment and infusion of chemotherapy drugs. They can also be used for intravenous infusion, parenteral nutrition, blood products, blood specimens, etc. PORT can be used for the infusion of all drugs of different natures.
A fully implantable infusion port, referred to as an infusion port, is a closed venous infusion system that is completely implanted subcutaneously and remains in the body for a long time. It includes a catheter part located in the superior vena cava and an injection seat implanted subcutaneously. It is a method of inserting a catheter through a puncture of the internal jugular vein or subclavian vein, allowing the catheter tip to enter the superior vena cava, inserting an infusion port seat in the subclavian fossa, and connecting the infusion port seat to the catheter. It is a long-term venous channel for intravenous infusion in patients. Depending on the implantation site, it can be divided into two types: chest wall infusion port and arm infusion port, which can be used for drug infusion, fluid replacement, nutritional support, blood transfusion, blood sample collection, etc.
Implant-grade PEEK materials have excellent physical and chemical properties, radiolucency and biocompatibility. They have successfully partially replaced metals and other high-performance resins in the fields of orthopedic tumors, plastic surgery, spine, knee joints and craniofacial repair.
AKSOPEEK Advantages
What are the advantages of AKSOPEEK infusion port over traditional infusion port
1. Good biocompatibility;
2. Low density, lighter and more comfortable to wear than metal PEEK infusion ports;
3. PEEK has good thermal insulation and is not easy to accumulate heat when bathing or sunbathing;
4. PEEK itself has antibacterial properties, so it is not easy to cause infection in the case of long-term implantation, thus reducing complications;
5. PEEK has good corrosion resistance and is not easy to react with the input liquid medicine;
6. PEEK has good radiation resistance and will not be affected by radiotherapy or chemotherapy rays.
Company advantages
1. As one of the earliest domestic companies engaged in the research, development, and production of PEEK materials, Junhua pays great attention to the application of PEEK in the medical industry and has been committed to the research, development, and production of medical implant-grade PEEK materials. It can supply implant-grade PEEK material granules, rods, plates, and 3D printing PEEK filaments.
2. After 16 years of production and technology accumulation, the company started from the source of polymerization and established Shandong Junhao High Performance Polymer Co., Ltd. to develop polymer implantable PEEK raw materials.

3. A GMP refining production workshop was established in accordance with the production standards for medical implant materials, and refining and purification equipment for implant-grade AKSOPEEK polymerization was developed, which effectively solved the problem of excessive heavy metal ion content in implant PEEK materials.
4. The company started pilot production of implantable-grade PEEK materials in 2020. In the same year, it conducted third-party tests on various biological aspects of the materials in accordance with ISO10993 testing standards, and all test results met the requirements.
5. In 2021, Junhua sent the first batch of mass-produced implantable-grade AKSOPEEK to a third party for inspection in accordance with the national requirements for implantable PEEK materials “YY/T 0660-2008 Standard Specification for Polyetheretherketone (PEEK) Polymers for Surgical Implants”, and obtained a complete third-party test report on implantable PEEK materials to ensure that the material can be effectively replaced in terms of physical and chemical properties and biology.
Implant-grade PEEK: As the name implies, PEEK materials that can be used to prepare “implant devices” are called implant-grade PEEK. This type of material is commonly used in orthopedic implant consumables (such as intervertebral fusion devices, ligament repair anchors, joint interface screws), neurosurgery patches (such as artificial skulls, maxillofacial bones), and cardiovascular products (such as heart valves, pacemaker housings, etc.). In recent years, modified PEEK materials have begun to be widely used in oral implants, traumatic bone plates and other products with high mechanical performance requirements. In addition to meeting the biocompatibility of medical-grade PEEK, implant-grade PEEK should also have more stringent biosafety requirements, such as systemic toxicity, genotoxicity, carcinogenicity, blood compatibility, and implantation reactions, and must also comply with the requirements of “YY/T 0660-2008 Standard Specification for Polyetheretherketone (PEEK) Polymers for Surgical Implants”.
AKSOPEEK
China’s implant-grade PEEK materials mainly rely on imports. Based on market feedback and national R&D project needs, Junhua has launched the domestic implant-grade PEEK material brand AKSOPEEK series of products.
Based on the present and looking to the future. In 2018, Changzhou Junhua Medical officially launched the implantable PEEK project. At present, it has obtained complete third-party reports on biology, physicochemistry, aging, etc. In order to better standardize implantable PEEK, it has added implantable AKSOPEEK series grades.
1) AKSOPEEK Natural : Implant-grade PEEK pure material
2) AKSOPEEK HA : Hydroxyapatite improves the biocompatibility of implantable PEEK materials
3) AKSOPEEK CF : Chopped carbon fiber implant grade PEEK material
4) AKSOPEEK LCF : Continuous carbon fiber implant grade PEEK material
Application Scenario
The domestically produced medical implant-grade PEEK material AKSOPEEK has excellent physical and chemical properties, and its radiotransparency, biocompatibility and human bone matching are no less than similar foreign products, achieving import substitution of PEEK materials in the fields of orthopedic tumors, plastic surgery, spine, knee joints and craniofacial repair.


What is PEEK?
PEEK and its modified composite materials show excellent performance under high temperature and high humidity conditions. PEEK have excellent chemical corrosion resistance, self-lubricating, and wear-resistant properties, so it has become the most ideal material to replace copper alloys and ordinary rubber. Compared with them, PEEK has longer service life which can reduce equipment operating costs, improve equipment operating reliability.
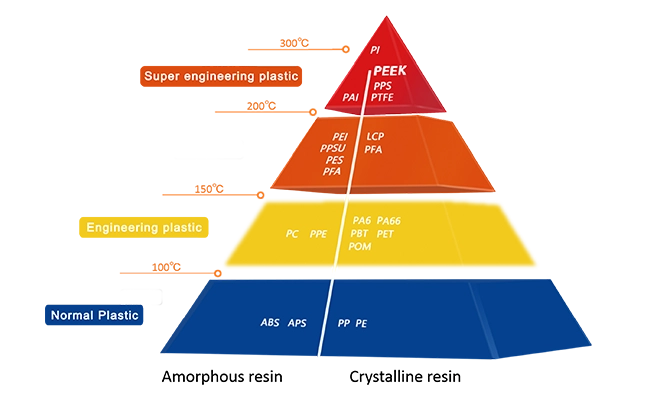

High Temperature Performance
PEEK’s performance can remain stable in the temperature range from -196°C to 260°C. Under extreme working conditions, it can withstand 300″C for a short time. The performance of PEEK is still reliable even in high temperatures of several kilometers underground work environment.

High Strength and Wear Resistance
Compared with other plastics, PEEK is resistant to compression, impact and also have good fatigue resistance. PEEK is durable in mechanical equipment and wear-resistant environments.

Double the Service Life
PEEK sealing solutions can improve the part service life. Some domestic chemical companies use the PEEK5600FE20 polytetrafluoroethylene modified material designed by Junhua to replace the original material, the service life of the wear parts can even be extended by 1.3-3 times.

Corrosion Resistance
PEEK is chemically inert, and has no reaction to most acids, oils, greases and all other organic and inorganic solvents or enzymes, and can still maintain good strength and dimensional stability.

Self-lubricating
PEEK has low friction coefficient, low running resistance, can be used without oil in some working conditions. The equipment is cleaner, thus improving process reliability, improving energy efficiency and saving costs.

More dom in Design
Injection molds can be used for rapid injection molding of large quantities of complex special-shaped parts, and the cost is low compared to machining.
Contact Us
Common PEEK Grades and Properties
1. PEEK5600G (brown-gray/khaki) is made from pure polyetheretherketone resin and offers the best toughness and impact resistance of all PEEK grades. Pure PEEK can be sterilized using convenient methods (steam, dry heat, ethanol, and gamma-rays), and the raw materials used in its manufacture comply with EU and US FDA food compatibility regulations. These characteristics make it widely used in the medical, pharmaceutical, and food processing industries.
2. PEEK5600LF30 (black) The addition of PTFE, graphite, and carbon fiber makes PEEK5600LF30 a bearing-grade plastic. Its superior tribological properties (low coefficient of friction, wear resistance, and high peak pressure limit) make it an ideal material for this class of friction applications.
3. PEEK5600GF30 (Brown-Gray): This material is a reinforced grade of plastic filled with 30% glass fiber. It offers superior rigidity, creep resistance, and dimensional stability compared to PEEK, making it ideal for structural parts. It can withstand fixed loads for extended periods at high temperatures. If PEEK5600GF30 is used as a sliding component, its suitability should be carefully verified, as the glass fiber can scratch mating surfaces.
4. PEEK5600CF30 (Black): This material is reinforced with 30% carbon fiber. It offers superior mechanical properties (higher elastic modulus, mechanical strength, and creep resistance) and wear resistance compared to PEEK5600GF30. Furthermore, carbon fiber-reinforced plastic has 3.5 times the thermal conductivity of unreinforced PEEK, dissipating heat more quickly from the bearing surface.
Mechanical Properties
| Item | Test Standard or Instrument |
Unit | PEEK5600G 100% PEEK |
PEEK5600GF30 PEEK+30% glass fiber |
PEEK5600CF30 PEEK+30% carbon fiber |
PEEK5600LF30 PEEK+30% (carbon fiber +graphite+PTFE) |
PEEK5600FE20 PEEK+20%PTFE |
|---|---|---|---|---|---|---|---|
| Tensile Strength (23℃) | ISO 527 | MPa | 95 | 175 | 250 | 145 | 70 |
| Tensile Modulus (23℃) | ISO 527 | GPa | 3.8 | 11 | 23 | 12.5 | / |
| Elongation at Break (23℃) | ISO 527 | % | 35 | 2.0 | 1.5 | 2.2 | / |
| Flexural Strength (23℃) | ISO 178 | MPa | 155 | 235 | 350 | 220 | 118 |
| Flexural Modulus (23℃) | ISO 178 | GPa | 3.5 | 10 | 21 | 11 | / |
| Charpy Impact Strength (unnotched) | ISO 179/1U | kJ/m2 | No break | 55 | 45 | 32 | No break |
| Cantilever Beam Impact Strength (notched) | ISO 180/A | kJ/m2 | 4 | 6 | 6.5 | 4 | 6 |
Thermal Properties
| Item | Test Standard or Instrument |
Unit | PEEK5600G 100% PEEK |
PEEK5600GF30 PEEK+30% glass fiber |
PEEK5600CF30 PEEK+30% carbon fiber |
PEEK5600LF30 PEEK+30% (carbon fiber +graphite+PTFE) |
PEEK5600FE20 PEEK+20%PTFE |
|---|---|---|---|---|---|---|---|
| Melting Point | ISO11357 | ℃ | 343 | 343 | 343 | 343 | 343 |
| Glass Transition Temperature | ISO11357 | ℃ | 143 | 143 | 143 | 143 | 150 |
| Heat Deflection Temperature | ISO 75A/B | 1.8MPa. ℃ | 152 | 315 | 315 | 293 | 150 |
| Thermal Expansion Coefficient | ASTM D696 | ppm K-1 | 45 | 22 | 15 | 22 | 70 |
| Thermal Conductivity | ISO /CD22007-4 | W/(m·K) | 0.29 | 0.32 | 0.95 | 0.86 | / |
Electrical Properties
| Item | Test Standard or Instrument |
Unit | PEEK5600G 100% PEEK |
PEEK5600GF30 PEEK+30% glass fiber |
PEEK5600CF30 PEEK+30% carbon fiber |
PEEK5600LF30 PEEK+30% (carbon fiber +graphite+PTFE) |
PEEK5600FE20 PEEK+20%PTFE |
|---|---|---|---|---|---|---|---|
| Dielectric Strength (2mm) | IEC 60243-1 | kV/mm | 20 | 19 | / | / | 19 |
| Dielectric Constant | IEC 62631 | – | 3.0 | 3.3 | / | / | 2.7 |
| Surface Resistivity | GB/T31838.3 | Ω | 1015 | 1014 | / | / | 1015 |
| Volume Resistivity | IEC 62631 | Ω·cm | 1015 | 1015 | 105 | 106 | / |
Other Properties
| Item | Test Standard or Instrument |
Unit | PEEK5600G 100% PEEK |
PEEK5600GF30 PEEK+30% glass fiber |
PEEK5600CF30 PEEK+30% carbon fiber |
PEEK5600LF30 PEEK+carbon fiber +graphite+PTFE |
PEEK5600FE20 PEEK+20%PTFE |
|---|---|---|---|---|---|---|---|
| Color | / | / | Natural | Natural | Black | Black | Natural |
| Melt Flow Index (400℃, 2.16kg) | ISO 1133 | g/10min | 6-10 | 2-5 | 1-3 | 2-5 | / |
| Density | ISO 1183 | g/cm3 | 1.30±0.02 | 1.50±0.02 | 1.40±0.02 | 1.44±0.02 | 1.41±0.02 |
| Water Absorption (23℃, 24Hrs) | ISO 62-1 | % | 0.07 | 0.05 | 0.04 | 0.05 | 0.15 |
| Rockwell Hardness | / | HRR | 118 | 119 | 121 | 108 | 113 |
| Flammability Rating | UL 94 | / | V-0 | V-0 | V-0 | V-0 | / |
| Coefficient of Friction | ASTM D3702 | 100N-120rpm | 0.30-0.38 | 0.38-0.46 | 0.15-0.25 | 0.18-0.30 | 0.1-0.2 |
*This data is for reference and not a guarantee. For a more detailed data sheet, please contact our company’s technical department.
Service
Junhua offers one-stop PEEK solutions from design to after-sales, ensuring quality, innovation, and customer satisfaction.
Design and processing
Junhua offers injection molding, machining, and extrusion solutions to optimize design and cost.
Material selection
Junhua specializes in PEEK with multiple grades and recommends the best engineering plastics for your needs.
Measurement
Junhua uses high-precision instruments to provide customers with accurate drawings.
Sampling
Junhua supplies samples for testing and refines designs until approval for mass production.
Mass Production
Junhua controls raw materials, processes, and inspections to ensure consistent product quality.
Quality Control
Junhua follows ISO9001 standards and provides full inspection reports as required.
Improve
Junhua continuously optimizes designs and processes through improvement systems and case experience.
After-sales Service
Junhua offers complete after-sales support to resolve technical issues and protect customer interests.

